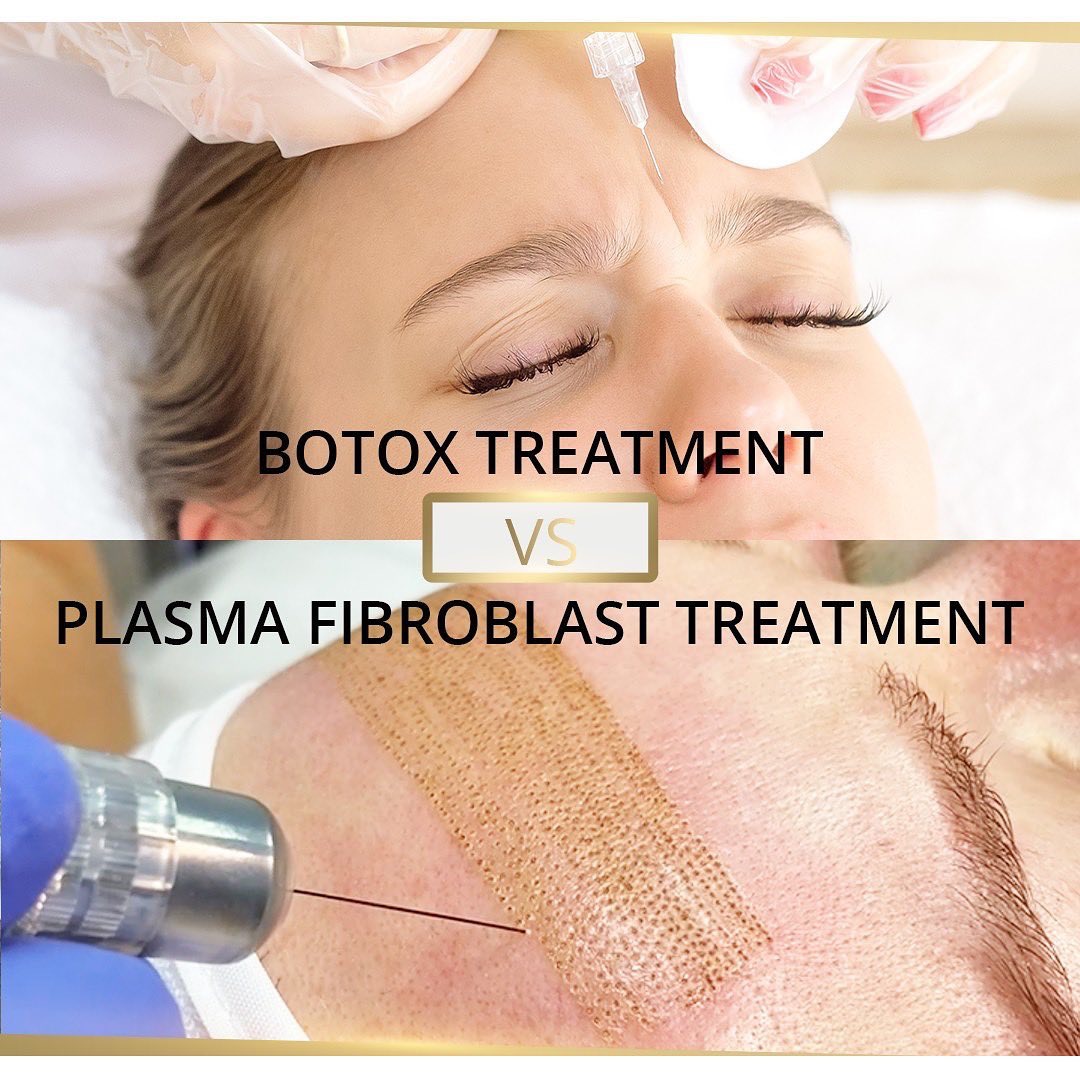
The Clinical Relevance of Collagen Loss After 35
In aesthetic dermatology, chronological aging intersects with environmental damage, hormonal shifts, and cellular senescence — but one molecular change underlies it all: progressive collagen degradation.
After the age of 35, the structural decline in collagen synthesis and quality becomes clinically significant. This article reviews the pathophysiology of collagen loss, the age-related decline in fibroblast activity, and evidence-based strategies to reactivate dermal remodeling — particularly relevant for aesthetic professionals working with advanced regenerative treatments.
Collagen: Structure, Function, and Age-Related Changes
Collagen is the primary structural protein in the dermis, predominantly types I and III, synthesized by dermal fibroblasts. Together with elastin and glycosaminoglycans (GAGs), it forms the extracellular matrix (ECM) responsible for skin firmness, elasticity, and tensile strength.
Key Facts:
- Type I collagen constitutes ~80% of dermal collagen and provides tensile strength.
- Type III collagen (~15%) is associated with pliability and wound healing.
- Collagen has a half-life of 15 years — but synthesis slows with age.
What Happens After 35? A Molecular and Cellular View
After age 35, the skin undergoes:
- Fibroblast senescence
- Reduced proliferative and secretory capacity
- Increased expression of senescence-associated secretory phenotype (SASP) factors, contributing to chronic low-grade inflammation (inflammaging)
- Enzymatic degradation of ECM
- Increased activity of matrix metalloproteinases (MMP-1, MMP-3, MMP-9)
- MMPs break down mature collagen fibers, while synthesis fails to compensate
- Decline in growth factor responsiveness
- Reduced sensitivity to TGF-β, FGF, and PDGF, impairing collagen synthesis
- Chronic UV exposure and oxidative stress further impair receptor signaling
- Hormonal shifts
- Estrogen deficiency post-35 accelerates collagen loss, especially in perimenopausal and menopausal women
- Estrogen regulates MMPs, collagen gene expression, and fibroblast activityClinical Manifestations of Collagen Decline
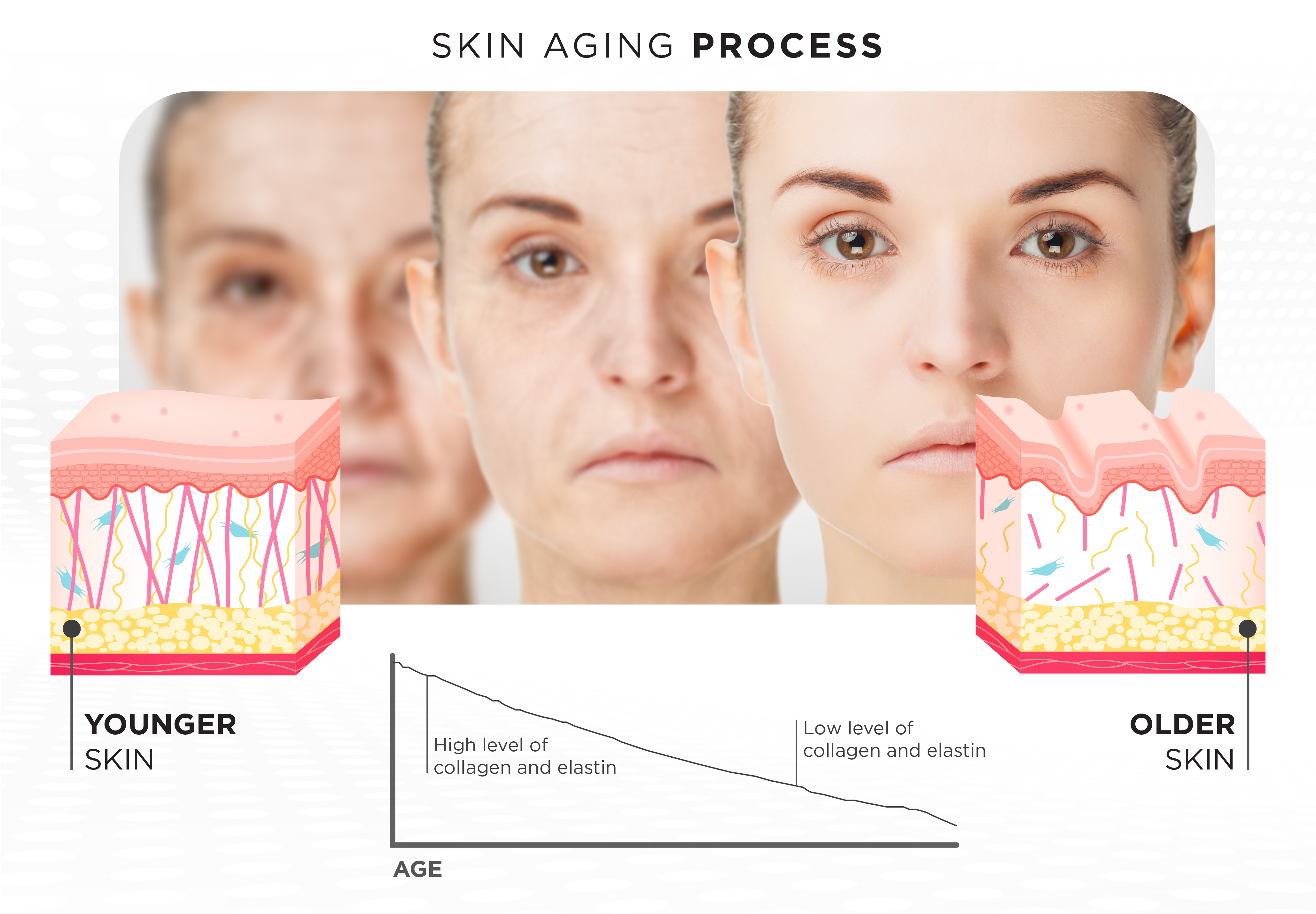
By age 40, the average adult has lost approximately 20–25% of dermal collagen, manifesting as:
- Skin thinning and laxity
- Delayed wound healing
- Fine lines and deepening rhytids
- Loss of dermal hydration due to ECM breakdown
- Volume loss due to reduced dermal support
Evidence-Based Methods to Stimulate Collagen Remodeling
To effectively counteract collagen decline, interventions must target fibroblast stimulation, controlled dermal injury, and regenerative signaling. Here's what the evidence supports:
1. Microneedling (Collagen Induction Therapy)
Mechanism:
- Controlled mechanical injury induces microchannels reaching the papillary and reticular dermis
- Triggers hemostasis, followed by platelet degranulation and growth factor release (TGF-β, PDGF, EGF)
- Activates fibroblast migration, proliferation, and ECM synthesis
Clinical Outcomes:
- Increased collagen I and III density (histologically confirmed)
- Improved skin texture, firmness, and scar remodeling
- Safe across all Fitzpatrick types
Recommended protocol:
- 3–6 sessions, 4–6 weeks apart, combined with peptide serums or PRP for enhanced outcomes
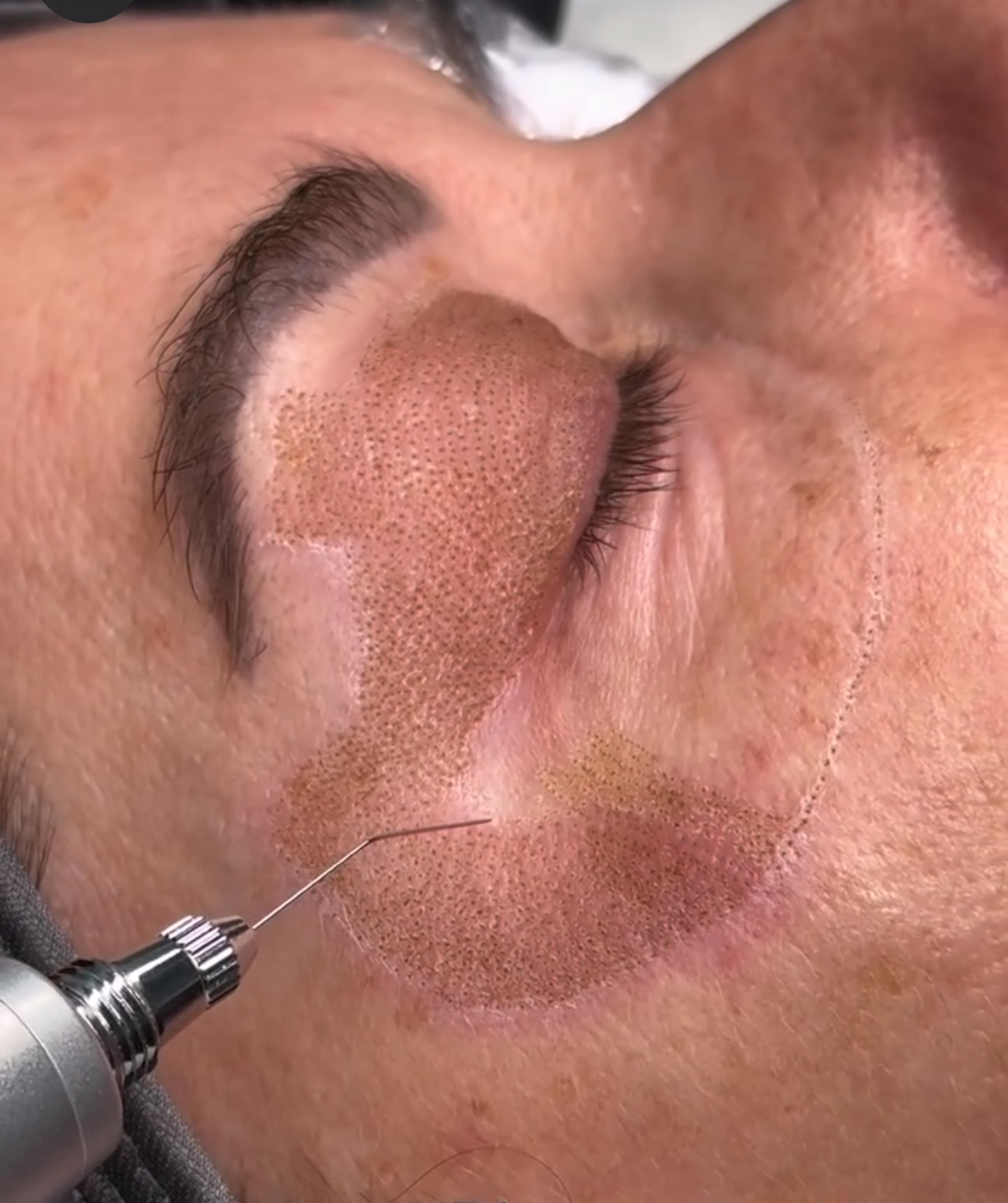
2. Plasma Energy (Plasma Fibroblast Therapy)
Mechanism:
- Non-contact ionization of atmospheric gas produces plasma arc
- Causes targeted epidermal ablation and controlled dermal heat injury
- Induces wound healing cascade and fibroblast activation through thermal shock proteins (HSPs)
Clinical Outcomes:
- Enhanced skin tightening and superficial resurfacing
- Effective for periorbital lines, upper eyelids, and small laxity zones
- Minimal downtime when applied with correct protocols
Precaution:
- Not suitable for darker phototypes without specialized training due to risk of PIH
3. Injectable and Topical Bio-activators
a. Platelet-Rich Plasma (PRP):
- Autologous source of growth factors (PDGF, VEGF, IGF-1)
- Stimulates fibroblasts and angiogenesis
- Best when combined with microneedling or fractional laser
b. Polynucleotides & Biostimulators (e.g., PDRN, poly-L-lactic acid):
- Reorganize ECM and promote neocollagenesis
- Suitable for advanced aging and structural loss
c. Topicals:
- Retinoic acid: Promotes fibroblast proliferation and collagen gene transcription
- Vitamin C (L-ascorbic acid): Co-factor in collagen synthesis; photoprotective
- Peptides (e.g. Matrixyl, Copper peptides): Signal fibroblast activity and inhibit MMPs
Conclusion: A Strategic Approach to Collagen Preservation
Understanding the biology of collagen degradation is essential for designing intelligent anti-aging strategies.
A proactive approach that combines mechanical stimulation, biological regeneration, and topical support can significantly delay visible aging and improve long-term skin integrity.
For the modern aesthetic professional, collagen management is not cosmetic — it’s clinical skin preservation.
Discover how advanced techniques like Plasma Fibroblast and Microneedling stimulate collagen remodeling, tighten tissue, and support fibroblast renewal — essential tools for every aesthetic professional committed to results-driven, science-based practice.




















%202.JPG)